 Multiple Choice Questions
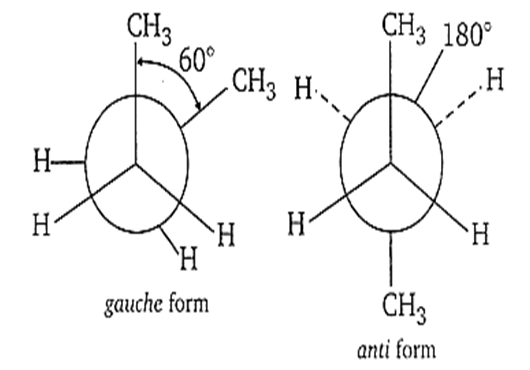
Multiple Choice QuestionsDihedral angle between two methyl groups of n-butane in the gauche and anti forms are
60°, 0°
0°, 60°
60, 180°
180°, 60°
C.
60, 180°
The angle between C-C and C-H bonds on adjacent carbon atoms in any conformation is called dihedral angle. Hence, the dihedral angle in case of gauche is 60°and in anti form is 180°.
The vapour pressure of 100g water reduces from 3000 Nm-2 to 2985 Nm-2 when 5g of substance 'X' is dissolved in it. Substance 'X' is
methanol
glucose
carbon dioxide
cannot predict
The complex ion which has no d-electrons in the central metal atom is
[MnO4]-
[Co(NH3)6]3+
[Fe(CN)6]3-
[Cr(H2O)6]3+
White P reacts with caustic soda, the products are PH3 and NaH2PO2. This reaction is an example of
hydrolysis
reduction
disproportionation
neutralisation
The solubility of saturated solution of Ag2CrO4 is s mol L-1. What is its solubility product?
4s3
s3
2s3
16s2
For a reaction, A+ B Product, the rate is given by,
r = k[A]1/2[B]2
What is the order of the reaction?
0.5
2
2.5
1
How long it will take to deposit 1.0 g of chromium when a current of 1.25 A flows through a solution of chromium (III) sulphate ? (Molar mass of Cr = 52)
1.24 min
1.24 h
1.24 s
None of these
If a be the edge length of the unit cell and r be the radius of an atom then for fee arrangement, the correct relation is
4a = r
4r = a
4r = a
4r =
A solid AB has the NaCl structure. If radius of cation A+ is 120 pm, the maximum possible value of the radius of the anion B- is
240 pm
60 pm
49.6 pm
290 pm
