 Multiple Choice Questions
Multiple Choice QuestionsThe chemical name for melamine is
1,3,5-Triamino-2,4,6-triazine
2,4,6-Triamino-1,3,5-triazine
2-Amino-1,3,5-triazine
2,4-Diamino-1,3,5-triazine
Bromine is added to cold dilute aqueous solution ofNaOH. The mixture is boiled. Which of the following statements is not true?
During the reaction bromine is present in four different oxidation states.
The greatest difference between the various oxidation states of bromine is 5.
On acidification ofthe final mixture bromine is formed.
Disproportionation of bromine occurs during the reaction.
B.
The greatest difference between the various oxidation states of bromine is 5.
On acidification, the final mixture give bromin.
5NaBrO3 + NaBrO3 + 6HCl 6NaCl +3Br2 + 3H2O
Thus, during the reaction, bromine is present in four different oxidation states i.e., zero in Br2, +1 in NaBrO, -1 in NaBr and +5 in NaBrO3. The greatest difference between various oxidation states of bromine is 6 and not 5. On acidification of the final mixture, Br2 is formed and disproportionation of Br2 occurs during the reaction giving BrO-, Br- and Br ions.
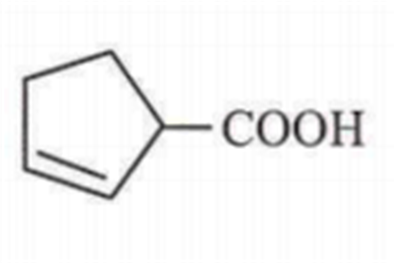
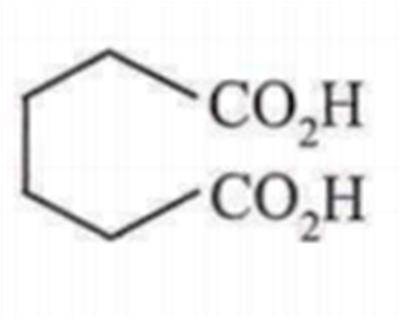
Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E. Compound Eon further treatment with aqueous KOH yields compound F. Compound F is
![]()
![]()
The compound which on reaction with aqueous nitrous acid at low temperature produces an oily nitrosoamine is
methyl amine
ethyl amine
diethyl amine
triethyl amine
Compound A (molecular formula C3H8O) is treated with acidified potassium dichromate to form a product B (molecular formula C3H6O). B forms a shining silver mirror on warming with ammoniacal silver nitrate. B when treated with an aqueous solution of H2NCONHNH2. HCl and sodium acetate gives a product C. Identify the structure of C.
CH3CH2CH=NNHCONH2
CH3-C(CH3)=NNHCONH2
CH3-C(CH3)=NCONHNH2
CH3CH2CH=NCONHNH2
Asssertion: CH3-C(COOC2H5)=CH-COOH is 3-carbethoxy-2-butenoic acid.
Reason : Principal functional group gets lowest number followed by double bond or triple bond.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Aldol condensation can be catalysed both by acids and bases.
Reason : -Hydroxy aldehydes or ketones readily undergo acid catalysed dehydration.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Benzene on heating with cone. H2SO4 gives benzenesulphonic acid which when heated with superheated steam under pressure gives benzene.
Reason : Sulphonation is a reversible process.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
