 Multiple Choice Questions
Multiple Choice QuestionsWhich has the maximum number of molecules among the following?
44 g CO2
48 g O3
8 g H2
8 g H2
According to the Bohr theory, which of the following transitions in the hydrogen atom will give rise to the least energetic photon?
n= 6 to n = 1
n = 5 to n = 4
n= 6 to n = 5
n= 6 to n = 5
Consider the following processes Δ H (kJ/mol)
1/2 A → +150
3B → 2 C + D -125
E + A → 2D +350
For B + D → E + 2C, ΔH will be
525 kJ/mol
-175 kJ/mol
-325 kJ /mol
-325 kJ /mol
Which of the following structures is the most preferred and hence of lowest energy of SO3 ?
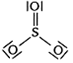
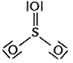
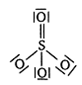

A bubble of air is underwater at temperature 15o C and the pressure 1.5 bar. If the bubbles rises to the surface where the temperature is 25o C and the pressure is 1.0 bar, what will happen to the volume of the bubble?
Volume will become greater by a factor of 1.6
Volume will become greater by a factor of 1.1
Volume will become smaller by a factor of 0.70
Volume will become smaller by a factor of 0.70
What is the value of electron gain enthalpy of Na+ if IE1 of Na = 5.1 eV?
-5.1 eV
-10.2 eV
+2.55 eV
+2.55 eV
Match (I) with list (II) for the compositions of substances and select the correct answer using the code given below the lists.
|
|
List I (substance) |
|
List II Composition |
|
A. |
Plaster of Paris |
1. |
CaSO4.2H2O |
|
B. |
Epsomite |
2. |
CaSO4. ½ H2O |
|
C. |
Kieserite |
3. |
MgSO4. 7H2O |
|
D. |
Gypsum |
4. 5. |
MgSO4.H2O CaSO4 |
|
A |
B |
C |
D |
|
3 |
4 |
1 |
2 |
|
A |
B |
C |
D |
|
2 |
3 |
4 |
1 |
|
A |
B |
C |
D |
|
1 |
2 |
3 |
5 |
|
A |
B |
C |
D |
|
1 |
2 |
3 |
5 |
The half -life of a substance in a certain enzyme catalysed reaction is 138 s. The time required for the concentration of the substance to fall from 1.28 mg L-1 is
414 s
552 s
690 s
690 s
Which of the following statements in incorrect?
pure sodium metal dissolves in liquid ammonia to give blue solution
NaOH reacts with glass to give sodium silicate
Aluminium reacts with excess NaOH to give Al(OH)3
Aluminium reacts with excess NaOH to give Al(OH)3
A 0.1 molal aqueous solution of a weak acid is 30% ionised. if Kf for water is 1.86oC/m, the freezing point of the solution will be
-18oC
-0.54oC
-0.36oC
-0.36oC