 Multiple Choice Questions
Multiple Choice Questions10-6 M NaOH is diluted 100 times. The pH of the diluted base is
between 7 and 8
between 5 and 6
between 6 and 7
between 10 and 11
Which one of the following complexes is outer orbital complex?
[Co(NH3)6]3+
[Mn(CN)6]4+
[Fe(CN)6]4-
[Ni(NH3)6]2+
A solution made by dissolving 40 g NaOH in 1000 g of H2O is
1 molar
1 normal
1 molal
None of these
Which of the following has the maximum number of unpaired electrons?
V3+
Fe2+
Mn2+
Cu+
C.
Mn2+
The outer electronic configuration of the given ions is as
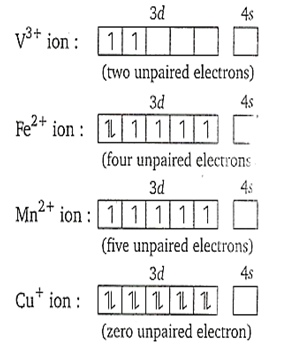
The correct statement with regard to and is
both H and H are equally stable
both H and H do not exist
H is more stable than H
H is more stable than H
