 Multiple Choice Questions
Multiple Choice QuestionsMaximum decarboxylation occurs in
CH3COOH
C6H5COOH
C6H5CH2COOH
CH3COCH2COOH
D.
CH3COCH2COOH
CH3COCH2COOH is a -keto acid. Thus, decarboxylation is maximum in a carboxylic acid containing an electron withdrawing group such as -CO or -COOH at the - carbon atom with respect to the -COOH group.
The correct increasing order of reactivity for the following molecules towards electrophilic aromatic substitution is
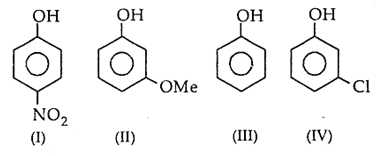
I < IV < II < III
I < IV < II < IV
I < III < II < IV
I < III < IV < II
The corrcet decreasing order of pKb is
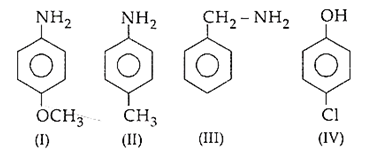
I > II > III > IV
III > IV > II > I
II > III > IV > I
IV > II > I > III
The correct decreasing order of pKa is
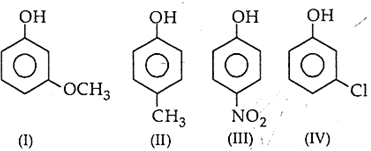
II > IV > I > III
IV > II > III > I
III > II > IV > I
IV > I > II > III
Assertion: Dehydration of alcohols always takes place in basic medium.
Reason : OH- is a better leaving group.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
Assertion : Toluene in presence of UV rays forms benzaldehyde.
Reason : Dichlorotoluene is formed as an intermediate.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
Assertion : -pleated sheet structure of protein shows maximum extension.
Reason : Intermolecular hydrogen bonding is present in them.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
Assertion : Fructose is a reducing sugar.
Reason : It has a ketonic group.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
Assertion : p-Nitrophenol gives more electrophilic substituted compound than m-methoxyphenol.
Reason : Methoxy group shows only negative I-effect.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
