 Multiple Choice Questions
Multiple Choice QuestionsIf the molecular wt. of Na2S2O3 and I2 are M1 and M2 respectively then what will be the equivalent weight of Na2S2O3 and I2 in the following reaction?
2S2O32- + I2 S4O62- + 2I-
M1 , M2
M1 , M2/2
2M1 , M2
M1 , 2M2
B.
M1 , M2/2
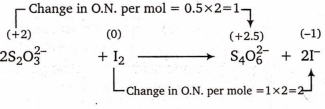
Equivalent mass of S2O32- = = M1 and equivalent mass of I2 =
A radioactive atom emits two particles and one particle successively. The number of neutrons in the nucleus of the product will be:
X - 4 - Y
X - Y - 5
X - Y - 3
X - Y - 6
The sp3d2 hybridisation of central atom of a molecule would lead to:
square planar geometry
tetrahedral geometry
trigonal bipyramidal geometry
octahedral geometry
The ease of nitration of the following three hydrocarbons follows the order:
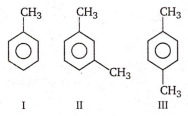
II = III = I
II > III > I
III > II > I
I = III > II
Among the alkenes which one produces tertiary butyl alcohol on acid hydration?
CH3-CH2-CH=CH2
CH3-CH=CH-CH3
(CH3)2C=CH2
CH3-CH=CH2
Which one of the following will show optical isomerism?
OH-CH2-CO2H
CH3-CH(OH)-CO2H
(CH3)2-CH-CO2H
(CH3)2-C(Cl)-CO2H
The ozone layer forms naturally by :
the interaction of CFC with oxygen
the interaction of UV radiation with oxygen
the interaction of IR radiation with oxygen
the interaction of oxygen and water vapour
