 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following is not condensation polymer?
Melamine
Glyptal
Dacron
Dacron
D.
Dacron
Condensation polymers are obtained by bifunctional molecules (monomers) with the elimination of smaller molecules whereas additional polymers are obtained from multiple bonds containing monomers. Neoprene is a polymer of chloroprene (CH2 =C(Cl)-C=CH2) so it is an addition polymer, not a condensation polymer.
In the following sequence of reactions,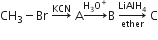
the end product is (C) is
acetone
methane
acetaldehyde
acetaldehyde
CH3CHO and C6H5CH2CHO can be distinguished chemically by
Benedict test
Iodoform test
Tollen's reagent test
Tollen's reagent test
Acetone is treated with an excess of ethanol in the presence of hydrochloric acid. The product obtained is.
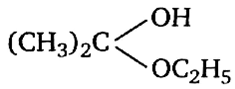
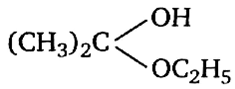
Among the following compounds, the one that is most reactive towards electrophilic nitration is
Benzoic acid
Nitrobenzene
toluene
toluene
The correct order of decreasing acid strength of trichloroacetic acid (A), trifluoroacetic acid (B), acetic acid (C) and formic acid (D) is
B > A> D > C
B > D > C > A
A> B > C > D
A> B > C > D
Which one of the following sets of monosaccharides forms sucrose?
Which of the following statements is false?
Artificial silk is derved from cellulose
Nylon-66 is an example of elastomer
The repeat unit in natural rubber isoprene
The repeat unit in natural rubber isoprene
