 Multiple Choice Questions
Multiple Choice QuestionsWhich characteristic property is not shown by carboxylic acid group ?
Alkyl group
-COOH group
ketonic group
none of these
C.
ketonic group
ketonic group characteristic property is not shown by carboxylic acid group .
The product of reaction between chloroform and acetone is used as :
insecticide
analgesic
hypnotic
war gas
The product formed by the reaction between acetylene and HOCl :
ethylene chloride
vinyl chloride
dichloro acetaldehyde
ethyledene chloride
The IUPAC name of 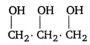 is :
is :
1 , 2 , 3 - propane tri-ol
3 - hydroxy butanoic acid
1 , 2 - ethane di-ol
3 - methyl butanol - 1
Which of the following statement is correct for ketone and acetaldehyde ?
Both reacts with HCN and forms hydrine
Both reacts with NaOH and forms polymer
both forms acid on oxidation and reduction
Both forms alcohol on reduction and oxidation
Aldehyde and ketone can be differentiated by :
sodium bi sulphite
Fehling solution
ammonia
sulphuric acid
The correct reactivity otder is :
CH3CHO > (CH3)2CO > CH3COC2H5
(CH3)2CO > CH3CHO > CH3COC2H5
CH3CHO > CH3COC2H5 > (CH3)2CO
CH3COC2H5 > CH3CHO > (CH3)2CO
The example of condensation polymerisation is :
formaldehyde - metaformaldehyde
acetaldenyde - para acetaldehyde
acetone - mesityl oxide
ethene - polyethene
The main product of heating of sodium formate at 360o C is :
CO
sodium oxalate
Na2CO3
None of the above
