 Multiple Choice Questions
Multiple Choice QuestionsAssertion : The solubility of a gas in a liquid increases with increase of pressure.
Reason : The solubility of a gas in a liquid is directly proportional to the pressure of the gas.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
A.
If both assertion and reason are true and reason is the correct explanation of assertion.
This is according to Henry's law which states that the solubility of a gas in given volume of a liquid at a particular temperature is directly proportional to the pressure of gas above the liquid. m p, m = kp where k= Henry's constant.
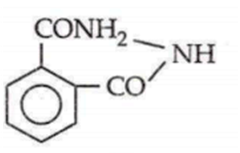
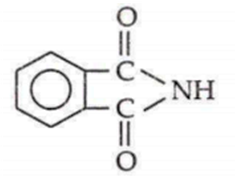
If phthalic acid is treated with NH3 and then it is first heated weakly then strongly, the final product formed is


Which is correct example of condensation polymer?
Nylon, Buna-S
Teflon, Buna-N
Nylon 6,6, Dacron
Neoprene, Buna-S
But-1-ene
The product in the above reaction is
CH3CH2CH2CH2OH
CH3CH2CH(OH)CH3
CH2=CH2CH(OH)-CH3
CH3-CH=C(OH)-CH3
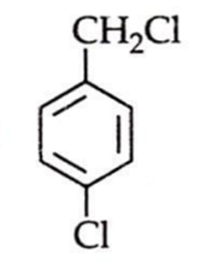
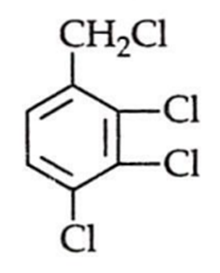
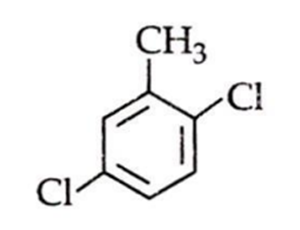
An aromatic compound C7H6Cl2 (A), gives AgCl on boiling with alcoholic AgNO2 solution and yields C,HOCI on treatment with sodium hydroxide. (A) on oxidation gives monochlorobenzoic acid. The compound (A) is:

Assertion: HC= C- is more stable than H2C CH-
Reason : HC C- has more s-character than HC= CH-
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
