 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following is a favourable factor for cation formation ?
Low ionisation potential
High electron affinity
High electronegativity
Small atomic size
Which of the following statements is not an essential feature of an optically active molecule ?
It will rotate the plane of polarised light
It will have a non-superimposable mirror image
It will have no element of symmetry
It will have an asymmetric carbon atom
The reagent which could distinguish between 1-hexyne and 1-hexene is :
Ag
KMnO4
Br2 in CCl4
H2SO4
Choose the correct reagent required to carry out the transformation :
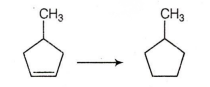
Zn + conc. HCl
conc. H2SO4
Li then H2O
H2/Pt
Ozone layer is being depleted. This is due to :
NO (nitric oxide) emission from supersonic jets
chlorofluoro carbon used as aerosols
Both (a) and (b)
None of the above
A.
NO (nitric oxide) emission from supersonic jets
Main reason of O3 layer depletion is the release of chlorofluorocarbon compounds, called freons and emission of nitric oxide (NO) from supersonic jets
NO + O3 NO2 + O2
CF2Cl2 Cl + CF2Cl
ClO + O3 ClO + O2
ClO + O Cl + O2
The chlorine free radicals are continuously regenerated and causes the breakdown of ozone.
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution :
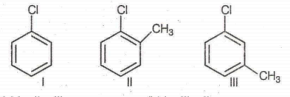
I < II < III
I < III < II
III < II < I
II < III < I
The oxygen carrying pigment , oxyhaemocyanin containing two copper ions is diamagnetic , because :
the two copper ions are in + 1oxidation state
one copper ion is in + 1oxidation state while other is in + 2 oxidation state
of the strong antiferromagnetic interactions between two copper ions
of the ferromagnetic interactions between the two copper ions
