 Multiple Choice Questions
Multiple Choice QuestionsWhich ofthe following is true regarding teflon ?
It is a linear, unbranched polymer of tetrafluoro ethylene
It has very high thermal stability
Polymer molecules are associated by strong dipole-dipole attraction
All of the above
D.
All of the above
Due to symmetry of F2C = CF2 and strength of C-F bond , branching does not take place and a linear chain teflon is always formed. High bond energy of C-F bond gives high thermal stability to polymer . Large electronegativity difference of 'C' and F makes C-F bond highly polar.
Cellulose has very high degree of hydrophilicity because of :
its amorphous nature
crystalline nature
presence of excessive voids in solid state
presence of many hydroxyl groups on the polymer back bone
By adding sodium dodecyl sulphate, during the electrophoresis of proteins, it is possible to :
determine a protein's isoelectric point
determine an enzyme's specific activity
determine the amino acid composition of the protein
presence a proteins native structure and biological activity
Which of the following aldoses gives an optically active compound upon reaction with warm dilute nitric acid ?
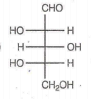
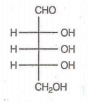
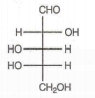
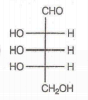
In the -helix , the hydrogen bonds
are roughly parallel to the axis of the helix
are roughly perpendicular to the axis of the helix
occur mainly between electronegative atoms of the R groups
occur only between some of the amino acids of the helix
Which one ofthe following antioxidant commonly used to increase the storage life of butter ?
Butylated hydroxy anisol
Sodium sulphite
Sodium metabisulphite
All of the above
The strongest acid among the following compound is :
HCOOH
CH3CH2CH(Cl)COOH
ClCH2CH2CH2COOH
CH3COOH
Phenol , when it first react with cone. sulphuric acid and then with cone. nitric acid gives :
nitrobenzene
2 , 4 , 6 - trinitrobenzene
o - nitrophenol
p - nitrophenol
During dehydration of alcohols to alkenes by heating with conc. H2SO4 , the initial step is :
formation of an este
protonation of alcohol molecule
formation of carbocation
elimination of water
