 Multiple Choice Questions
Multiple Choice QuestionsWhat is the maximum number of orbitals that can be identified with the following quantum numbers?
n=3, l =1, m1 = 0
1
2
3
3
A.
1
The value of n=3 and l =1 suggest that it is a 3p orbital while the value of m1 = 0 [magnetic quantum number] shows that the given 3p orbital is 3pz in nature.
Hence, the maximum number of orbitals identified by the given quantum number is only 1, i.e. 3pz.
Calculate the energy in joule corresponding to light of wavelength 45mm: (Planck's constant h=6.63 x10-34)Js; speed of light c= 3 x 108 ms-1)
6.67 x 1015
6.67 x 1011
4.42 x 10-15
4.42 x 10-15
Equal masses of H2, O2 and methane have been taken in a container of volume V at temperature 270 C in identical conditions. The ratio of the volumes of gases H2 : O2: CH4 would be
8:16:1
16:8:1
16:1:2
16:1:2
When 22.4 L of H2 (g) is mixed with 11.2 L of Cl2 (g), each of at STP, the moles of HCl (g) formed is equal to
1 mole of HCl (g)
2 moles of HCl (g)
0.5 mole of (g)
0.5 mole of (g)
Which of the following statement is correct for the spontaneous absorption of a gas?
For the reversible reaction,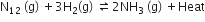
the equilibrium shifts in the forward direction
by increasing the concentration of NH3 (g)
by decreasing the pressure
by decreasing the concentrations of N2 (g) and H2(g)
by decreasing the concentrations of N2 (g) and H2(g)
For the reaction X2O4 (l) --> 2XO2 (g) ΔU = 2.1 kcal, ΔS = 20 cal K-1 at 300 K hence ΔG is
2.7 kcal
-2.7kcal
9.3 kcal
9.3 kcal
For a given exothermic reaction Kp and Kp' are the equilibrium constant at temperatures T1 and T2 respectively. Assuming that heat of reaction si constant in temperature range between T1 and T2 it is readily observed that
Kp> Kp'
Kp< Kp'
Kp = Kp'
Kp = Kp'
which of the following orders of ionic radii is correctly represented?
H- > H >H+
Na+ >F- >O2-
F- > O2->Na+
F- > O2->Na+
