 Multiple Choice Questions
Multiple Choice QuestionsThe correct order of magnitude of bond angles among the compounds CH4, NH3 and H2O is
CH4 > H2O < NH3
H2O < NH3 < CH4
NH3 < CH4 < H2O
NH3 < H2O < CH4
The dissociation equilibrium of a gas AB2 can be represented as
2AB2 (g) 2AB (g) + B2(g)
The degree of dissociation is 'x' and is small compared to 1. The expression relating the degree of dissociation (x) with equilibrium constant KP and total pressure p is
A buffer solution is prepared in which the concentration of NH3 is 0.30 M and the concentration of N is 0.20 M. If the equilibrium constant, Kb for NH3 equals 1. 8 x 10-5, what is the pH of this solution? (log 2. 7 =0. 43).
9.43
11.72
8.73
9.08
If a gas expands at constant temperature indicates that
kinetic energy of molecules decreases
pressure of the gas increases
kinetic energy of molecules remains as same
number of the molecules of gas increase
If n = 6, the correct sequence for filling of electrons will be
ns (n - 1)d (n - 2)f np
ns (n - 2)f np (n - 1)d
ns np (n - 1)d (n - 2)f
ns (n - 2)f (n - 1)d np
For the reaction, N2(g) + O2(g) 2NO (g), the equilibrium constant is K1. The equilibrium constant is K2 for the reaction, 2NO(g) + O2)g) 2NO2(g). What is K for the reaction, NO2(g) ?
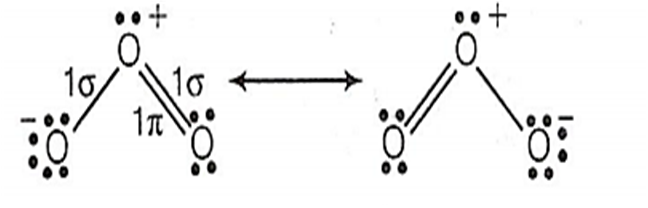
The angular shape of ozone molecule (O3) consists of
1 sigma and 2 pi bonds
2 sigma and 2 pi bonds
1 sigma and 1 pi bonds
2 sigma and 1 pi bonds
D.
2 sigma and 1 pi bonds
Single bond contains= 1 bond
Double bond contains = 1 bond + 1 bonds
Triple bond contains= 1 bond + 2 bonds
The molecule of O3 is bent with an angle 116.8° and equal O - O distance of 128 pm. The ozone molecule consists of 2 sigma and 1 pi bonds. The angular shape of molecule is represented as
An electron is moving in Bohr's fourth orbit. Its de-Broglie wave length is . What is the circumference of the fourth orbit?
2
4
