 Multiple Choice Questions
Multiple Choice QuestionsA diatomic gas at pressure P, compressed adiabatically to half of its volume, what is the final pressure?
(2)1.4P
P/(2)1.4
(2)5/3P
P/(2)5/3
Choose the correctly paired gaseous cation and its magnetic (spin only) moment (in B.M.)
Ti2+,3.87 B.M.
Cr2+, 4.90 B.M.
Co3+,3.87 B.M.
Mn2+,4.90 B.M.
The equilibrium constant for the reaction,
HI(g) is Kc
Equilibrium constant for the reaction 2HI(g) H2(g) + I2(g) will be
1/Kc
1/(Kc)2
2/Kc
2/(Kc)2
In O3 molecule, the formal charge on the central O-atom is
0
-1
-2
+1
D.
+1
Lewis gave the structure of O, molecule as
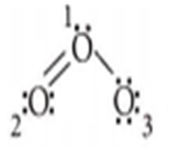
Using the relation,
Formal charge = [Total no. of valence electrons in the free atom] - [Total no. of non-bonding (lone pair) electrons]- [Total no. of bonding (shared) electrons]
The formal charge on central O -atom i.e., no. 1
=6-2- -(6)=+1
Which ofthe following statements is incorrect?
Li+ has minimum degree of hydration.
The oxidation state of K in KO2 is + 1.
Na is used to make a Na/Pb alloy.
MgSO4 is readily soluble in water.
