 Multiple Choice Questions
Multiple Choice QuestionsThe emission spectrum of hydrogen discovered first and the region of the electromagnetic spectrum in which it belongs, respectively are
Lyman, ultraviolet
Lyman, visible
Balmer, ultraviolet
Balmer, visible
As per de-Broglie's formula a macroscopic particle of mass 100 g and moving at a velocity of 100 cm s-1 will have a wavelength of
6.6 × 10-29 cm
6.6 × 10-30 cm
6.6 × 10-31 cm
6.6 × 10-32 cm
For one mole of an ideal gas,the slope of V vs T curve at constant pressure of 2 atm is X L mol-1 K-1.The value of the ideal universal gas constant 'R' in terms of X is
X L atm mol-1 K-1
L atm mol-1 K-1
2X L atm mol-1 K-1
2 X atm L-1 mol-1 K-1
At a certain temperature the time required for the complete diffusion of 200-mL of H2 gas is 30 min. The time required for the complete diffusion of 50 mL of O2 gas at the same temperature will be
60 min
30 min
45 min
15 min
The correct order of decreasing H-C-H angle in the following molecule is
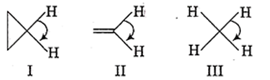
I > II > III
II > I > III
III > II > I
I > III > II
During the emission of a positron from a nucleus, the mass number of the daughter element remains the same but the atomic number
is decreased by 1 unit
is decreased by 2 units·
is increased by 1 unit
remains unchanged
β- emission is always accompained by
formation of antineutrino and α-particle
emission of α-particle and γ-ray
formation of antineutrino and γ-ray
formation of antineutrino and positron
Four gases P, Q, R and S have almost same values of 'b' but their 'a' values (a, b are van der Waals' constants) are in the order Q < R < S < P. At a particular temperature, among the four gases, the most easily liquefiable one is
P
Q
R
S
The compound that will have a permanent dipole moment among the following is
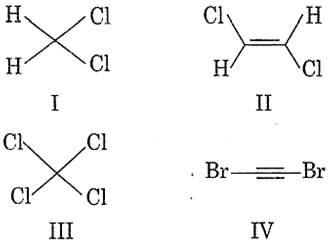
I
II
III
IV
A.
I
Symmetrical molecules and symmetrical trans- alkene have a net dipole moment zero. CH2Cl2 is not a symmetrical molecule, thus it will have a permanent dipole.

μ1 + μ2 - μ3 - μ4 = 0 ; μ3 and μ4 > μ1 and μ2. Thus μnet ≠ 0
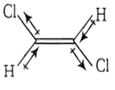
Symmetrical trans-alkene μnet ≠ 0

μ1 + μ2 = μ3 + μ4 ; μnet = 0
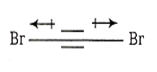
μnet = 0
The correct order of decreasing length of the bond as indicated by the arrow in the following structures is
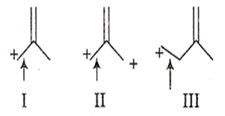
I > II > III
II > I > III
III > II > I
I > III > II
