Multiple Choice Questions
Multiple Choice QuestionsCupric compounds are more stable than their cuprous counterparts in solid state. This is because
the endothermic character of the 2nd IP of Cu is not so high
size of Cu2+ is less than Cu+
Cu2+ has stable electronic configuration as compared to Cu+
the lattice energy released for cupric compounds is much higher than Cu+
A.
the endothermic character of the 2nd IP of Cu is not so high
B.
size of Cu2+ is less than Cu+
D.
the lattice energy released for cupric compounds is much higher than Cu+
Electronic configuration of cuprous (Cu+) and cupric (Cu2+) ions are as follows-
Cu+ = [Ar] 3d104s0
Cu2+ = [Ar] 3d94s0
Electronic configuration of Cu+ is more stable as compared to Cu2+. It is because in Cu2+(small size) the nuclear charge is sufficient to hold 27 electrons whereas in Cu+ such condition is not possible.
Further, the IInd IP of Cu is not very high as compared to its Ist IP. Consequently a large amount of lattice energy is released for cupric compounds as compared to Cu+ compounds.
The correct statement regarding the following compounds is
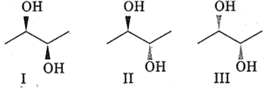
all three compounds are chiral
only I and II are chiral
I and III are diastereomers
only I and III are chiral
Among the following compounds, the one(s) that gives (give) effervescence with aqueous NaHCO3 solution is (are)
I and II
I and III
Only II
I and IV
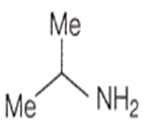
An amine C3H9N reacts with benzene sulphonyl chloride to form a white precipitate which is insoluble in aq. NaOH. The amine is
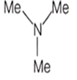
![]()
![]()
The number of amino acids and number of peptide bonds in a linear tetrapeptide (made of different amino acids) are respectively
4 and 4
5 and 5
5 and 4
4 and 3
In DNA, the consecutive deoxynucleotides are connected via
phosphodiester linkage
phosphomonoester linkage
phosphotriester linkage
amide linkage
The reaction of aniline with chloroform under alkaline conditions leads to the formation of
phenylcyanide
phenylisonitrile
phenylcyanate
phenylisocyanate
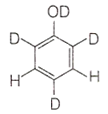
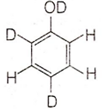
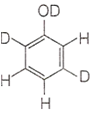
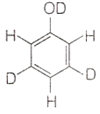
When phenol is treated with D2SO4 /D2O, some of the hydrogens get exchanged. The final product in this exchange reaction is