 Multiple Choice Questions
Multiple Choice QuestionsA gas such as carbon monoxide would be most likely to obey the ideal gas law at
high temperatures and low pressures
low temperatures and high pressures
high temperatures and high pressures
high temperatures and high pressures
If Avogadro number NA, changed from 6.022 x 1023 mol-1 this would change
the definition of mass in units of grams
the mass of one mole of carbon
the ratio of chemical species to each other in the balanced equation
the ratio of chemical species to each other in the balanced equation
What is the pH of the resulting solution when equal volumes of 0.1 M NaOH and 0.01 M HCl are mixed?
12.65
2.0
7.0
7.0
Which one of the following pairs of the solution is not an acidic buffer?
HClO4 and NaClO4
CH3COOH and CH3COONa
H2CO3 and Na2PO4
H2CO3 and Na2PO4
20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample? (Atomic weight of Mg =24)
75
96
60
60
The heat of combustion of carbon to CO2 is -395.5 kJ/mol. The heat released upon the formation of 35.2 g CO2 from carbon and oxygen gas is
-315 kJ
+315 kJ
-630 kJ
-630 kJ
The formation of the oxide ion O2- (g), from oxygen atom requires first an exothermic and then an endothermic step as shown below,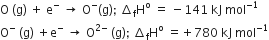
Thus, the process of formation of O2- in the gas phase is unfavourable even though O2- is isoelectronic with neon. It is due to the fact that
electron repulsion outweighs the stability gained by achieving a noble gas configuration
O- ion has comparatively smaller size that oxygen atom
oxygen is more electronegative
oxygen is more electronegative
What is the mass of precipitate formed when 50 mL of 16.9% solution of AgNO3 is mixed with 50 mL of 5.8% NaCl solution?
(Ag = 107.8, N = 14, O = 16, Na = 23, Cl = 35.5)
28 g
3.5 g
7 g
7 g
Which is the correct order of increasing energy of the listed orbitals in the atom of titanium?
3s 4s 3p 3d
4s 3s 3p 3d
3s 3p 3d 4s
3s 3p 3d 4s