 Multiple Choice Questions
Multiple Choice QuestionsAn aqueous solution of which of the following compounds is the best conductor of electric current?
Acetic acid C2H4O2
Hydrochloric acid, HCl
Ammonia, NH3
Ammonia, NH3
What is the mole fraction of the solute in a 1.00 m aqueous solution?
0.177
1.770
0.0354
0.0354
The number of water molecules is maximum in
18 molecules of water
1.8 g of water
18 g of water
18 g of water
In which of the following pairs, both the species are not isostructural?
SiCl4.PCl4+
Diamond, carbide
NH3, PH3
NH3, PH3
Which of the following statement is correct for a nucleophile?
Nucleophile is s Lewis acid
Ammonia is a nucleophile
Nucleophiles attack low electrons density sites
Nucleophiles attack low electrons density sites
The number of structural isomers possible from the molecular formula C3H9N is
4
5
2
2
Two possible stereo-structures of CH3CHOH.COOH, which are optically active, are called
diastereomers
atropisomers
enantiomers
enantiomers
C.
enantiomers
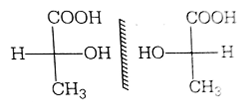
Gadolinium belongs to 4f series. Its atomic number is 64. which of the following is the correct electronic configuration of gadolinium?
[Xe]4f8 6d2
[Xe]4f95s1
[Xe]4f7 5d16s2
[Xe]4f7 5d16s2
