 Multiple Choice Questions
Multiple Choice QuestionsThe hybridization involved in complex [Ni(CN)4]2- is (Atomic number of Ni = 28)
dsp2
sp3
d2sp3
d2sp3
A.
dsp2
[Ni(CN)4]2-
Let oxidation state of Ni in [Ni(CN)4]2- is x.
x - 4 = - 2
Or
x= 2
Now, Ni2+ = [Ar] 3d8 4so![]()
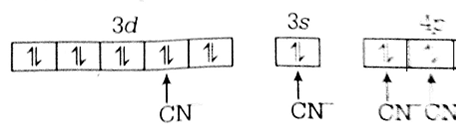
Which of the statements given below is incorrect?
Cl2O7 is an anhydride of perchloric acid
O3molecule is bent
ONF is isoelectronic with NO2
ONF is isoelectronic with NO2
Strong reducing behaviour of H3PO4 is due to
the presence of one -OH group and two P-H bonds
high electron gain enthalpy of phosphorous
the high oxidation state of phosphorus
the high oxidation state of phosphorus
Which of the following reaction (s) can be used for the preparation of alkyl halides?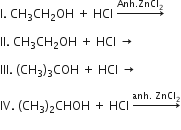
I, III and IV
I and II
Only IV
Only IV
In an SN1 reaction on chiral centres, there is
100% racemisation
inversion more than retention leading to partial racemization
100% retention
100% retention
A reaction of a carbonyl compound with one of the following reagents involves nucleophilic addition followed by the elimination of water. The reagents is
a Grignard reagent
hydrazine in presence of feebly acidic solution
hydrocyanic acid
hydrocyanic acid
Method by which aniline cannot be prepared is
hydrolysis phenyl isocyanide with the acidic solution
degradation of benzamide with bromine in alkaline solution
reduction of nitrobenzene with H2/Pd in ethanol
reduction of nitrobenzene with H2/Pd in ethanol
Which one of the following esters gets hydrolysed most easily under alkaline conditions?
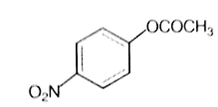
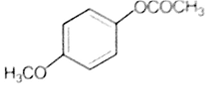
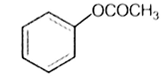

The reaction of phenol with chloroform in the presence of dilute sodium hydroxide finally introduces, which one of the following functional group?
-CH2Cl
-COOH
-CHCl2
-CHCl2
