 Multiple Choice Questions
Multiple Choice QuestionsThe correct order of basic strength of the following are
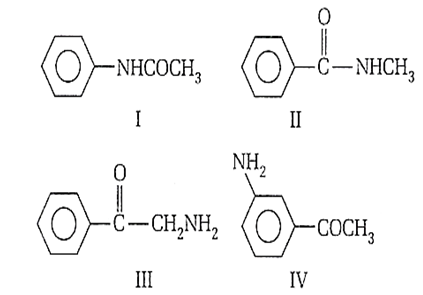
I > II > IV > III
IV > III > II > I
II > III > IV I
III > IV > II > I
If for a given substance, melting point is TB and freezing point is TA then correct variation of entropy by graph between entropy change and temperature is
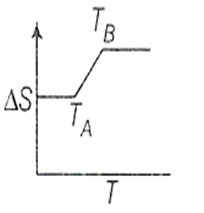
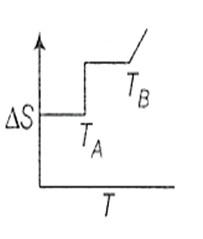

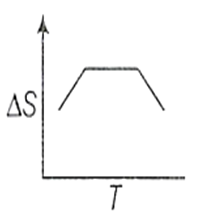
B.

For a pure substance TA and TB are same. Above to this temperature, entropy will increase and below this temperature entropy remains constant.
for the rreaction,
Ag2O(s) 2Ag(s) + O2 (g) are 30.56 kJ mol-1 and 66.00 JK-1 mol-1 respectively. The temperature at which the free energy change for the reaction will be zero is
3528 K
463 K
73 K
144 K
Ig Ag+ + 2NH3 Ag (NH3 ; K1 = 1.7 x 107
Ag+ + Cl- AgCl; K2 = 5.4 x 109
Then, for AgCl + 2NH3 [Ag(NH3)2]+ + Cl- equilibrium constant will be
0.68 x 10-3
5.2 x 10-17
0.31 x 10-2
3.1 x 10-2
For the following equilibrium (omitting charges)
I. M + Cl MCl, Keq = 1
II. MCl + Cl MCl2, Keq = 2
III. MCl2 +Cl MCl3 ,Keq = 3
IV. M + 3Cl MCl3 , Keq = K
then relationship between K,
K =
All of the above
R - CH2 - CH2 - OH can be converted into RCH2CH2COOH by the following sequence of steps.
PBr3, KCN, H2 /Pt
PBr3, KCN, H3O+
HCN, PBr3, H3O+
KCN, H3O+
0.001 mole of [Co(NH3)5(NO3) (SO4)] was passed through a cation exchanger and the acid coming out of it required 20mL of 0.1 M NaOH for neutralisation. Thus, the complex is
[Co(NH3)5(NO3)] SO4
[Co(NH3)5(SO4)]NO3
[Co(NH3)5] NO3.SO4
None of the above
In the complexes [Fe(H2O)6]3+ , [Fe(CN)6]3-, [Fe(C2O4)3]3- and [FeCl6]3-, more stability is
[FeCl6]3-
[Fe(C2O4)3]3-
[Fe(H2O)6]3+
[Fe(CN)6]3-
A student made the following observations in the laboratory.
I. Clean copper metal did not react with 1 molar Pb(NO3)2 solution
II. Clean lead metal dissolved in a 1 molar AgNO3 solution and crystals of Ag metal appeared
III. Clean silver metal did not react with 1 molar Cu(NO3)2 solution
The order of decrease in reducing character of three metals is
Pb, Cu, Ag
Pb, Ag, Cu
Cu, Ag, Pb
Cu, Pb, Ag
In order to prepare one litre 1N solution of KMnO4, how many grams of KMnO4 are required, if the solution to be used in acid medium for oxidation?
128g
41.75 g
31.60 g
62.34 g
