 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following represents the correct order of decreasing number of S = O bonds?
H2SO4 > H2SO3 > H2S2O8
H2S2O8 > H2SO3 > H2SO4
H2S2O8 > H2SO4 > H2SO3
H2SO3 > H2S2O8 > H2SO4
Given that the reduced temperature, ; the reduced pressure, and the reduced volume,
Thus, it can be said that the reduced equation of state may be given as
When KMnO4 acts as an oxidising agent and ultimately forms Mn MnO2, Mn2O3 and Mn2+ then the number of electrons, transferred in each case respectively are
1, 3, 4, 5
3, 2, 1, 4
1, 5, 3, 7
4, 3, 2, 1
In acidic medium, dichromatic ion oxidises ferrous ion to ferric ion. If the gram molecular weight of potassium dichromate is 294 g its gram equivalent weight (in grams) is
24.5
49
125
250
Find out the correct stereoisomeric product for the following reaction,
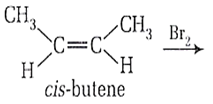
d-form
l- from
meso-form
racemic mixture
Point out the correct statement.
Below 710C, C is better reducing agent than CO
Below 710°C, CO is better reducing agent than C
Below 710C, CO is an oxidising agent
Below 710C, CO, is a reducing agent
Which of the following atomic and physical properties of hydrogen is false?
Hydrogen > Deuterium > Tritium; (melting point/ K)
Hydrogen < Deuterium < Tritium; (boiling point/ K)
Hydrogen < Deuterium < Tritium; (density /gL-1)
Hydrogen > Deuterium > Tritium; (% relative abundance)
A.
Hydrogen > Deuterium > Tritium; (melting point/ K)
Among all the given options, option 1 is incorrect. The correct order is as follows-
| Property | Tritium | Deuterium | Hydrogen |
| Melting point (K) | 20.62 | 18.5 | 13.9 |
| Boiling point (K) | 24.9 | 23.5 | 20.4 |
| Density (g/L) | 0.27 | 0.18 | 0.09 |
| Relative abundance (%) | 10-15 | 0.016 | 99.984 |
Eutrophication of a lake means, it
is low is nutrients
is high in nutrients
has a high temperature
has excess amount of organic matter
