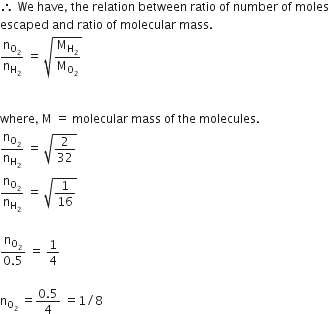Multiple Choice Questions
Multiple Choice QuestionsThe addition of a catalyst during a chemical reaction alters which of the following quantities ?
Internal energy
Enthalpy
Activation energy
Activation energy
Predict the correct order among the following.
long pair-lone pair> bond pair-bond pair> lone pair>bond pair
bond pair-bond pair> lone pair-bond pair > lone pair -lone pair
lone pair -bond pair > bond pair- bond pair> lone pair -lone pair
lone pair -bond pair > bond pair- bond pair> lone pair -lone pair
Two electrons occupying the same orbital are distinguished by
Magnetic quantum number
Azimuthal quantum number
Spin quantum number
Spin quantum number
The electronic configuration of Eu (Atomic no.63), Gd (Atomic no. 64) and Tb (Atomic no. 65) are
[Xe]4f6 5d1 6s2, [Xe] 4f7 5d1 6s2 and [Xe] 4f9 6s2
[Xe] 4f6 5d1 6s2 , [Xe] 4f7 5d1 6s2 and [Xe] 4f8 5d1 6s2
[Xe] 4f7 6s2, [Xe] 4f7 5d1 6s2 and [Xe] 4f9 6s2
[Xe] 4f7 6s2, [Xe] 4f7 5d1 6s2 and [Xe] 4f9 6s2
Which one of the following characteristics is associated with adsorption?
ΔG, ΔH and ΔS all are negative
ΔG and ΔH are negative but ΔS is positive
ΔG and ΔS are negative but ΔH is positive
ΔG and ΔS are negative but ΔH is positive
The pair of an electron in the given carbanion, is present in which orbital?
is present in which orbital?
sp3
sp2
sp
sp
Consider the molecules CH4, NH3 and H2O. Which of the given statement is false?
The H-O-H bond angle in H2O is larger than the H-C-H bond angle in CH4.
The H-O-H bond angle in H2O is smaller than the H-N-H bond angle in NH3.
The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3
The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3
The correct thermodynamic conditions for the spontaneous reaction at all temperatures is
In which of the following options the order of arrangement does not agree with the variation of the property indicated against it?
B<C<N<O (increasing first ionisation enthalpy)
I<Br<Cl<F(increasing electron gain enthalpy)
Li<Na<K<Rb (increasing metallic radius)
Li<Na<K<Rb (increasing metallic radius)
Equal moles of hydrogen and oxygen gases are placed in a container with a pin-hole through which both can escape. What fraction of the oxygen escapes in the time required for one-half of the hydrogen to escape?
1/4
3/8
1/2
1/2
D.
1/2
we have given,
a number of moles of hydrogen and not equal.
not equal.