 Multiple Choice Questions
Multiple Choice QuestionsWhen excess of NaOH solution is added to aqueous solution of iodine, the colour of solution becomes
blue
yellow
colourless
pale green
Which of the following statements is incorrect regarding the reaction?
CH3CHO + [Ag(NH3)2] + OH- CH3COO- + Ag
The equivalent weight of CH3CHO is 22
Three moles of OH- are required in the above D reaction
CH3CHO is an oxidising agent
Reduction of [Ag(NH3)2] occurs
In the reaction,
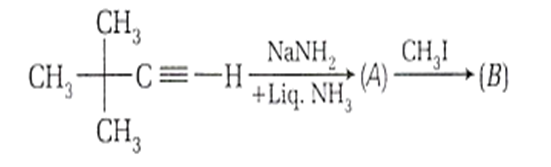
How many electron donating groups are attached with the carbon atom of unsaturated part of the product 'B' ?
Two
Three
Four
None of these
A.
Two

There are two electron donating grops present at the carbon of unsaturated part of product 'D', i.e. C C.
The magnitude of screening effect depends upon the number of
inner electrons
outer electrons
bond order
Both (a) and (b)
Duma's method involves the determination of nitrogen content in the organic compound in the form of
NH3
N2
NaCN
(NH4)2SO4
Which of the following statement(s) is not correct?
Suspended particulate matter is an important pollutant released by diesel vehicles
Soot particles (size < 5) cause fibrosis of the lung living
H2SO4 particulates have size of 500- 1000 nm
Photochemical smog is formed by oxides of sulphur, smoke and dust particles
Solid NaHCO3 will be neutralised by 40.0 mL of 0.1 M H2SO4 solution. What would be the weight of solid NaHCO3 in gram?
0.672 g
6.07 g
17 g
20 g
For the cell reaction,
Pb + Sn2+ Pb2+ + Sn
Given that, Pb Pb2+, E = 0.13 V
Sn2+ +2e- Sn, E = -0.14 V
What would be the ratio of cation concentration for which E = 0 ?
[CuCl4]2- exists while [CuI4]2- does not exist, because
I- is stronger reductant than Cl-
I- is weaker reductant than Cl-
I- is stronger oxidant than Cl-
None of the above
