 Multiple Choice Questions
Multiple Choice QuestionsThe freezing point depression of 0.001 m Kx[Fe(CN)6] is 7.1x 10-3 K. The value of x will be [Given, Kf = 1.86 KKg mol-1 for water].
2
4
3
1
Three ampere currernt was passed through an aqueous solution of an unknown salt of Pd for 1 h 2.977 g of Pdn+ was deposited at cathode.The value of n is:
(given at wf of Pd= 106.4)
6
5
4
3
The variation of adsorption of N, on charcoal with pressure at different constant temperature can be represented by :
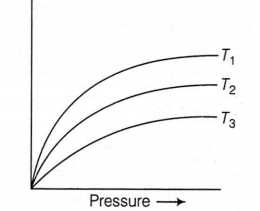
The correct increasing order of temperature
T1 , T2 and T3 is :
T1 < T2 < T3
T2 < T3 < T1
T3 < T2 < T1
T2 < T1 < T3
Reduction potential of some ions are given below:
Arrange them in decreasing order of oxidising power.
Ions Reduction : Cl I Br
Potential Ev = 1.19 V Ev = 1.741
Potential Ev / V
Cl > I < Br
I > Br < Cl
Br < I < Cl
Br < Cl < I
The coordination number of Fe[Fe(CN)6]4- , [Fe(CN)6]3- and [FeCl4]- respectively:
6 , 3 , 3
6 , 4 , 6
6 , 6 , 4
2 , 3 , 3
The metal which can displace all other metals from their salt solution among the following is:
Al
Zn
Cu
Fe
Yellow colour of aqueous solution of potassium chromate changes to orange if dil. H2SO4 is added. It indicates:
chromate ions are reduced
chromate ions are oxidised
monocentric complex is converted into dicentric complex
oxygen gets removed from chromate ions
There is a steam of neutrons with a kinetic energy of 0.0327 eV. If the half life of neutrons s 700 s, what fraction of neutrons will decay before they travel a distance of 10 km?
0.001
0.004
0.006
0.009
One of the hazardous explosion is the generation of 90Sr and its subsequent incorporation in bones. This nuclide has a half life of 28.1 years. Suppose one microgram was absorbed by a new born child. How much 90Sr will remain in his bones after 20 years?
0.061 µg
0.080µg
0.050µg
0.030µg
A.
0.061 µg
Mass of 90Sr at start , No = 1µg mass 90Sr after 20 years , Nt = ?
Time t=20 years
T1/2 = 28.1years
Half life of 90Sr
Calculation of decay constant , =
Substituting the values in the relation
X = log
0.0247 = log
On usual calculation , Nt = 0.061µg
