 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following reagents cannot be used for the given conversion?
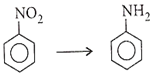
Sn-HCl
Fe-HCl
LiAlH4
Pd/C
C.
LiAlH4
With LiAlH4 nitroarenes give azo compounds.
2C6H5NO2 C6H5 - N = N - C6H5
The reaction,
C6H5ONa + CO2 +H2O C6H5OH + NaHCO3 suggests that
phenol is a stronger acid than carbonic acid
carbonic acid is a stronger acid than phenol
water is a stronger acid than phenol
None of these.
Which one of the following does not give white precipitate with acidified silver nitrate solution?

CH2 = CH - Cl
CH2 = CH - CH2 - Cl
Both (a) and (b)
Oil used as frothing agent in froth-floatation process is
pine oil
mustard oil
coconut oil
olive oil
Which amine amongst the following will answer positively the carbylamine test?
C6H5 - NH - CH3
![]()
C6H5 - NH - C4H9
C6H5 - N(C2H5)2
Assertion : Phenol is more acidic than ethanol.
Reason : Phenoxide ion is resonance stabilised.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
Assertion : 2-Methyl-1, 3-butadiene is the monomer of natural rubber.
Reason : Natural rubber is formed through anionic addition polymerisation.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
