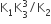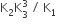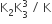Multiple Choice Questions
Multiple Choice QuestionsAn example of a sigma bonded organometallic compound is :
Ruthenocene
Grignard's reagent
Ferrocene
Ferrocene
B.
Grignard's reagent
Grignard's reagent i.e., RMgX is σ-bonded organometallic compound.
Which of the following is dependent on temperature?
Molality
Molarity
Mole fraction
Mole fraction
In the electrochemical cell :
Zn|ZnSO4(0.01M)||CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is the relationship between E1 and E2?
(Given, RT/F= 0.059)
E1= E2
E1< E2
E1> E2
E1> E2
In which pair of ions both the species contain S – S bond?
S2O72–, S2O32–
S4O62–, S2O32–
S2O72–, S2O82–
S2O72–, S2O82–
The correct order of the stoichiometries of AgCl formed when AgNO3 in excess is treated with the complexes: CoCl3.6NH3, CoCl3.5NH3, CoCl3.4NH3 respectively is
1 AgCl, 3 AgCl, 2 AgCl
3 AgCl, 1 AgCl, 2 AgCl
3 AgCl, 2 AgCl, 1 AgCl
3 AgCl, 2 AgCl, 1 AgCl
Correct increasing order for the wavelengths of absorption in the visible region for the complexes of Co3+ is
[Co(en)3]3+, [Co(NH3)6]3+, [Co(H2O)6]3+
[Co(H2O)6]3+, [Co(en)3]3+, [Co(NH3)6]3+
[Co(H2O)6]3+, [Co(NH3)6]3+, [Co(en)3]3+
[Co(H2O)6]3+, [Co(NH3)6]3+, [Co(en)3]3+
A first-order reaction has a specific reaction rate of 10–2 s–1. How much time will it take for 20 g of the reactant to reduce to 5 g?
238.6 second
138.6 second
346.5 second
346.5 second
Ionic mobility of which of the following alkali metal ions is lowest when aqueous solution of their salts are put under an electric field?
Na
K
Rb
Rb
The equilibrium constants of the following are.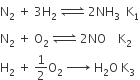
The equilibrium constant (K) of the reaction: