 Multiple Choice Questions
Multiple Choice QuestionsConsider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below :
Then the species undergoing disproportionation is
HBrO
Br2
Which one of the following conditions will favour maximum formation of the product in the reaction,
Low temperature and high pressure
Low temperature and low pressure
High temperature and low pressure
High temperature and high pressure
The bond dissociation energies of X2, Y2 and XY are in the ratio of 1 : 0.5: 1. ΔH for the formation of XY is –200 kJ mol–1. The bond dissociation energy of X2 will be
200 kJ mol-1
100 kJ mol-1
400 kJ mol-1
800 kJ mol-1
The correction factor ‘a’ to the ideal gas equation corresponds to
Density of the gas molecules
Volume of the gas molecules
Forces of attraction between the gas molecules
Electric field present between the gas molecules
Which one is a wrong statement?
Total orbital angular momentum of electron in 's' orbital is equal to zero
An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers
The value of m for dz2 is zero
The electronic configuration of N atom is

D.
The electronic configuration of N atom is

According to Hund's Rule of maximum multiplicity, the correct electronic configuration of N-atom is
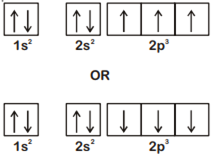
Magnesium reacts with an element (X) to form an ionic compound. If the ground state electronic configuration of (X) is 1s2 2s2 2p3, the simplest formula for this compound is
Mg2X3
MgX2
Mg3X2
Mg2X
The correct order of atomic radii in group 13 elements is
B < Al < In < Ga < Tl
B < Al < Ga < In < Tl
B < Ga < Al < In < Tl
B < Ga < Al < Tl < In
Which oxide of nitrogen is not a common pollutant introduced into the atmosphere both due to natural and human activity?
N2O5
NO2
NO
N2O
Which of the following molecules represents the order of hybridisation sp2, sp2, sp, sp from left to right atoms?
HC≡C-C≡CH
CH2=CH-C≡CH
CH3-CH=CH-CH3
CH2=CH-CH=CH2
