 Multiple Choice Questions
Multiple Choice QuestionsA nucleus has 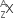 mass represented by M (A, Z). If Mp and Mn denote the mass of proton and neutron respectively and BE the binding energy (in MeV), then:
mass represented by M (A, Z). If Mp and Mn denote the mass of proton and neutron respectively and BE the binding energy (in MeV), then:
BE = [M(A,Z)-ZMp - (A-Z)Mn]c2
BE = [ZMp + (A-Z)Mn -M(A,Z)]c2
BE = [ZMp + AMn - M (A,Z)]c2
BE = [ZMp + AMn - M (A,Z)]c2
In the following circuit the output Y for all possible input A and B is expressed by the truth table:![]()
| A | B | Y |
| 0 | 0 | 0 |
| 0 | 1 | 0 |
| 1 | 0 | 0 |
| 1 | 1 | 1 |
| A | B | Y |
| 0 | 0 | 1 |
| 0 | 1 | 1 |
| 1 | 0 | 1 |
| 1 | 1 | 0 |
| A | B | Y |
| 0 | 0 | 1 |
| 0 | 1 | 1 |
| 1 | 0 | 1 |
| 1 | 1 | 0 |
| A | B | Y |
| 0 | 0 | 1 |
| 0 | 1 | 1 |
| 1 | 0 | 1 |
| 1 | 1 | 0 |
If the nucleus 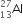 has a nuclear radius of about 3.6 fm, then
has a nuclear radius of about 3.6 fm, then  would have its radius approximately as:
would have its radius approximately as:
6.0 fm
9.6 fm
12.0 fm
12.0 fm
The total energy of an electron in the ground state of a hydrogen atom is -13.6 eV. The kinetic energy of an electron in the first excited state is:
3.4 eV
6.8 eV
13.6 eV
13.6 eV
Monochromatic light of frequency 6.0 x 1014 Hz is produced by a laser. The power emitted is 2 x 10-3 W. The number of photons emitted, on the average, by the source per second is:
5 x 1015
5 x 1016
5 x 1017
5 x 1017
The frequency of a light wave in the material is 2 x 10 Hz and wavelength is 5000 A. the refractive index of material will be:
1.40
1.50
3.00
3.00
In the energy band diagram of a material shown below, the open circles and filled circles denote holes and electrons respectively. The material is a/an,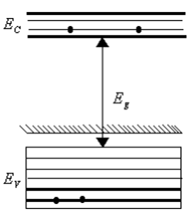
p-type semiconductor
insulator
metal
metal
A.
p-type semiconductor
The given figure represent p -type semiconductor as described below:
When one of the silicon atom (valence = 4) has been replaced by an atom of aluminum (valence = 3), the aluminum atom can bond covalently with only three silicon atoms, so there is now a 'missing' electron (a hole) in one aluminum -silicon bond with a small expenditure of energy, an electron can be torn from a neighboring silicon -silicon bond to fill this hole, thereby creating a hole in that bond similarly, an electron from some other bond can be moved to fill the second hole. In this way, the hole can migrate through the lattice.
The aluminium atoms are called an acceptor atom because it readily accepts an electron from neighbouring bond that is from the valence band of silicon. As figure suggest, the electrons occupy a localised acceptor state that lies within the energy gap, at an average energy interval Ea above the top of the valence band.
By adding acceptor atoms, it is possible to increase very greatly the number of holes in the valence band.
A 5 W source emits monochromatic light of wavelength 5000 A. When placed 0.5 m away, it liberates photoelectrons from a photosensitive metallic surface. when the source si moved to a distance of 1.0 m, the number of photoelectrons liberated will be reduced by a factor of:
4
8
16
16
Two radioactive substance A and B have decay constants 5λ and λ respectively. At t = 0 they have the same number of nuclei. The ratio of a number of nuclei of A to those of B will be  after a time interval:
after a time interval:
1/ 4λ
4λ
2λ
1/2λ
Monochromatic light of frequency 6.0 x 1014 Hz is produced by a laser. The power emitted is 2 x 10-3 W The number of photons emitted, on the average, by the source per second is:
5 x 1015
5 x 106
5 x 1017
5 x 1017
