(i) What type of isomers are [Co(NH3)5Br]SO4 and [Co(NH3)5SO4]Br.? Give a chemical test to distinguish between them.
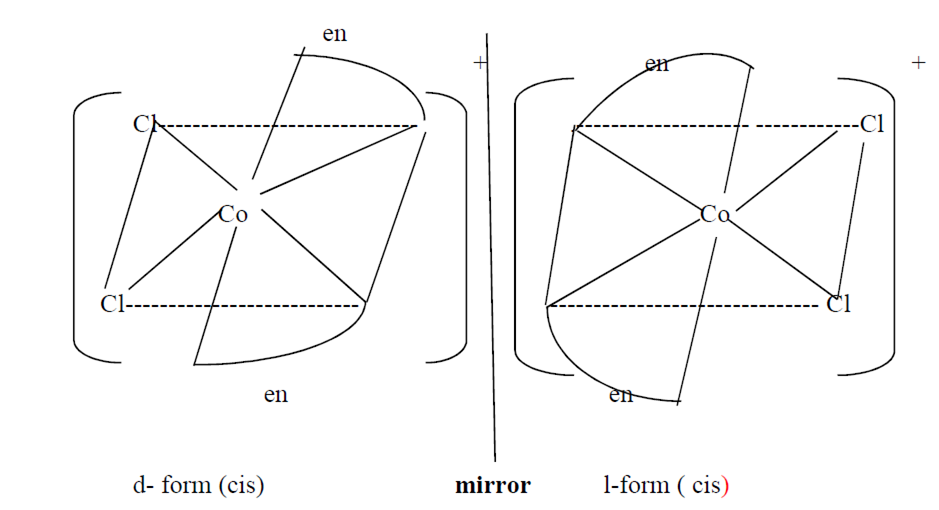
(ii) Write the structures of optical isomers of the complex ion [Co(en)2Cl2]+
i) Ionisation isomers
One of these is red-violet and forms a precipitate with BaCl2indicating that sulphate ion is outside the coordination sphere. The second one is red and does not form ppt. with BaCl2 but forms a ppt. of AgBr with AgNO3 indicating that bromide ion is outside the coordination sphere.
ii)