The melting point of pure silver and pure lead is 961°C and 327°C respectively. A molten mixture of the two metals on cooling at a constant temperature forms a eutectic mixture with a composition of approximately 5% silver and 95% lead at 305°C. Draw a schematic phase diagram for the silver-lead system. Label all the parts of the diagram. Indicate the number of components, phases and degrees of freedom present at the eutectic point.
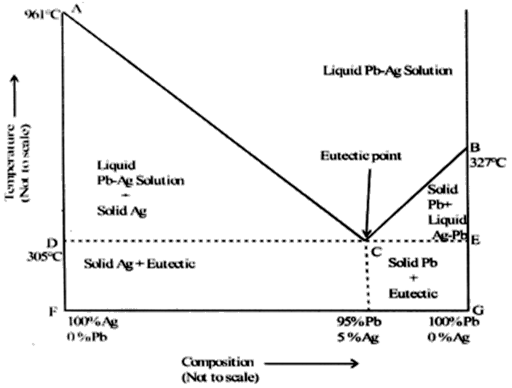
Point C is the eutectic point.
Temperature 303°C, composition 95% Pb and 5% Ag.
No. of component (C) at eutectic point = 2 (Pb and Ag)
No. of phases (P) at eutectic point = 3 (solid Ag, solid Pb and solution)
Degree of freedom (F) = C – P + 1
= 2 – 3 + 1 = 0
∴The system is non-variant and we need not specify any variable to define the system as both temperature and pressure are fixed.
380 Views

