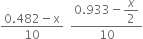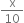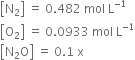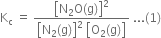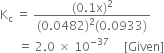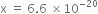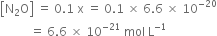Reaction between N2 and O2 takes place as follows:

If a mixture of 0.482 mol of N2 and 0.933 mol of O2 is placed in a 10 L reaction vessel and allowed to form N2O at a temperature for which Kc = 2·0 × 10–37, determine the composition of equilibrium mixture.
The given reversible chemical reaction is 2N
2(g) + O
2(g)

2N
2O(g)
Initial 0.482 mol 0.933 mol
At equi. 4.482 - x

x
Molar conc.
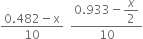
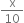
As
K = 2·0 × 10–37 is very small, this means that the amount of N2 and O2 reacted (x) is very very small. Hence, at equilibrium, we have
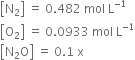
According to the law of chemical equilibrium,
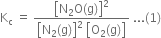
Putting the values in expression (1), we have,
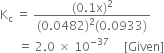
On solving
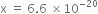
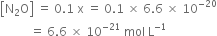
613 Views

 2N2O(g)
2N2O(g) x
x