Discuss the formation of N2 molecule on the basis of MO theory. Predict its:
(i) Bond order
(ii) Magnetic character.
The electronic structure of nitrogen atom is  Leaving out 4 electrons in the 1s orbital of two nitrogen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 10 electrons in nitrogen (N2) is as shown as below:
Leaving out 4 electrons in the 1s orbital of two nitrogen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 10 electrons in nitrogen (N2) is as shown as below: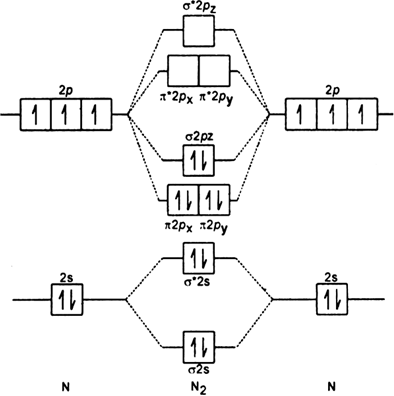
(i) Electronic configuration:
N2 : [KK (σ2s)2 (σ2s)2 ( 2px)1(σ2pz)2]
2px)1(σ2pz)2]
(i) Bond order : Here Nh = 8 and Na = 2
The two nitrogen atoms in nitrogen molecule are linked by three covalent bonds (i.e. a triple bond).
(ii) Magnetic character: Since all the electrons are paired, nitrogen is diamagnetic.
