What is the effect of temperature on the distribution of molecular velocities?
On increasing the temperature, the motion of the gas molecules becomes rapid and hence the value of most probable velocity also increases. As a result, the entire distribution curve becomes flatter and peak shifts to regions of higher velocities as shown in the figure.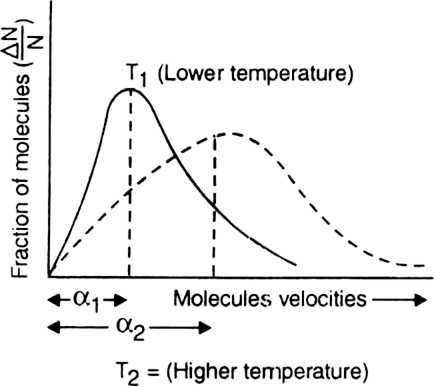
α1 = Most probable velocity at temp. T1
α2 = Most probable velocity at temp. T2
α2 > α1
