Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Hex-2-ene has the following cis and trans structures: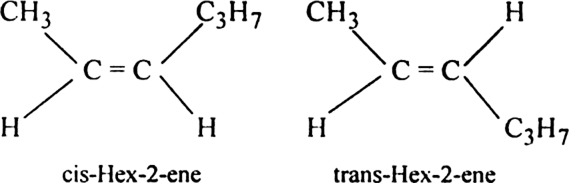
cis isomer possesses higher boiling point due to the greater magnitude of dipole-dipole interactions as compared to the trans isomer.
