Discuss the mechanism of electrophilic substitution reactions of benzene?
Typical reactions of benzene ring namely halogenation, nitration, sulphonation, and Friedel-Craft reaction are electrophilic substitutions.
Benzene ring serves as a source of electrons (nucleophile) due to the presence of electron cloud. The electrophilic reagent will attack the aromatic nucleus and the hydrogen atom of benzene is displaced by the electrophilic reagent. A substituted product is formed via intermediate carbonium ion formation.
Such reactions which are initiated by the electrophiles are called Electrophilic substitution reactions. The product formed is known as substituted product.
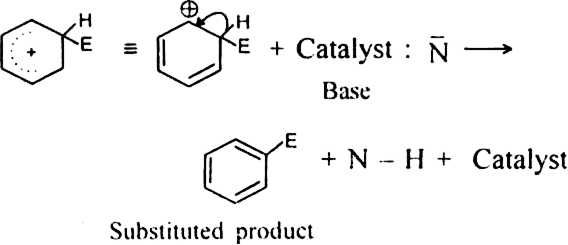
Let us consider a reaction of the attacking reagent E - N on the benzene in the presence of a catalyst. Substituted product is formed. 
Mechanism. It involves the following steps:
(i) Generation of an electrophile (E+): The function of the catalyst is to produce an electrophile from the attacking reagent E - N.
(ii) Formation of intermediate carbocation: The electrophile (E+) then is attacked by the n electrons of the benzene ring to form an intermediate carbocation. It is resonance stabilised.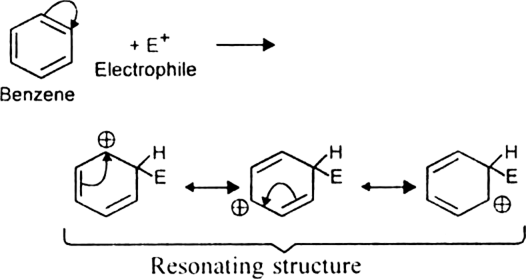
The resonance hybrid structure of the above resonance forms can be represented as
(iii) Catalyst-regenerating step.The base (Catalyst:N-) then removes the hydrogen ion from the intermediate carbocation and aromatization takes place forming the final product.