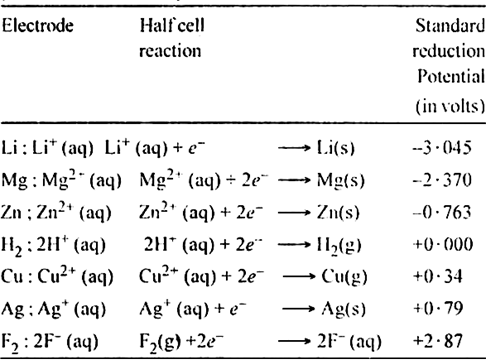
Applications:
(i) To predict the relative oxidising and reducing powers: Greater the reduction potential, more easily the substance is reduced and hence is a stronger oxidising agent. For example oxidising powers of halogens are F2 > Cl2 > Br2 > I2.
(ii) To predict whether a metal will react with acid to give H2 gas : Metals above hydrogen in the series displace hydrogen from acids.
(iii) To calculate the standard E.M.F. of the cell
(iv) To predict the spontaneity of any redox reaction: If E.M.F. of the cell is positive, it is spontaneous, otherwise not.
