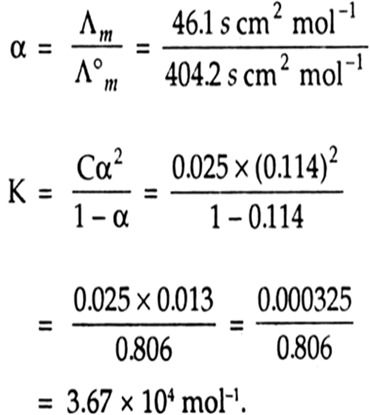
The molar conductivity of 0.025 mol L–1 methanoic acid is 46.1 S cm2 mol–1. Calculate its degree of dissociation and dissociation constant. Give λ°(H+) = 349.6 S cm2 mol–1 and λ° (HCOO– ) = 54.6 s cm2 mol–1.
Λ°m (HCOOH) = Λ°H+ + Λ°HCOO–
= 349.6 + 54.6
= 404.2 s cm2mol–1
Λ m= 46.1 s cm2 mol–1
225 Views

