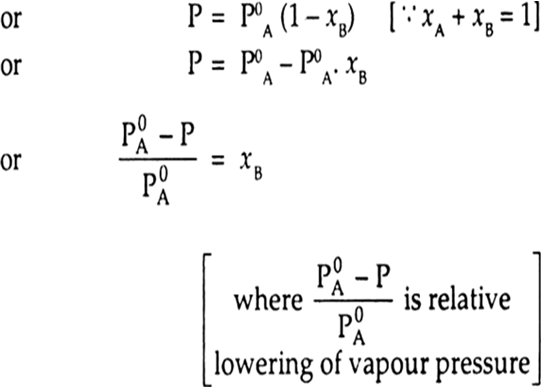
If the non volatile solute is present in the solution, the vapour pressure of the solution depends only upon the solvent. If the vapour pressure of the solution is P and the partial vapour pressure of solvent is PA then P = PA
(Because solute has no partial vapour pressure due to non-volatile nature)
or ![]()
where P0A is the vapour pressure of pure solvent and xA and xB are the mole fractions of solvent and solute respectively.