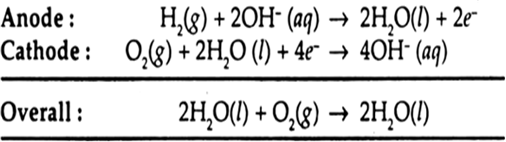What are fuel cells? Write reaction of a oxygen-hydrogen fuel cell. Write two advantages of the use of a hydrogen-oxygen fuel cell.
A device to convert chemical energy of fuel into electrical energy is called fuel cell.
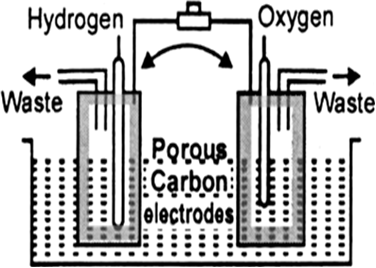
Hydrogen-oxygen fuel cell: The cell consists of three compartments separated from one another by porous electrode. The hydrogen gas is fed into one compartment and the oxygen gas is fed into another compartment. These gases then diffuse slowly through the electrodes and react with an electrolyte that is in the central compartment. The electrodes are made of a conducting material, such as graphite, with a sprinkling of platinum to act as a catalyst, and the electrolyte is an aqueous solution of a base. The reactions are
Advantages: (i) Fuel cells are efficient and free from pollution.
(ii) The only product in the reaction of fuel cell is water which can be removed and the astronauts of a spacecraft can drink it.
1138 Views