(a)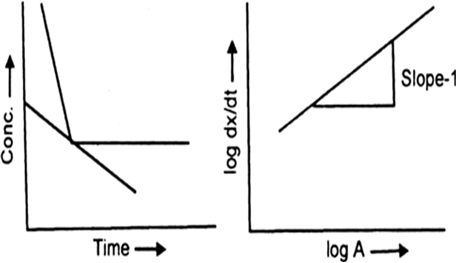
The rate i.e., dx / dt depends on the concentration of the reactant. Let dx / dt = k[A]1.
On taking log![]()
On plotting log dx / dt versus log A is always a straight line with slope equal to 1.
(b) Example of a first order reaction.
Decomposition of nitrogen pentoxide (N2O5)![]()
