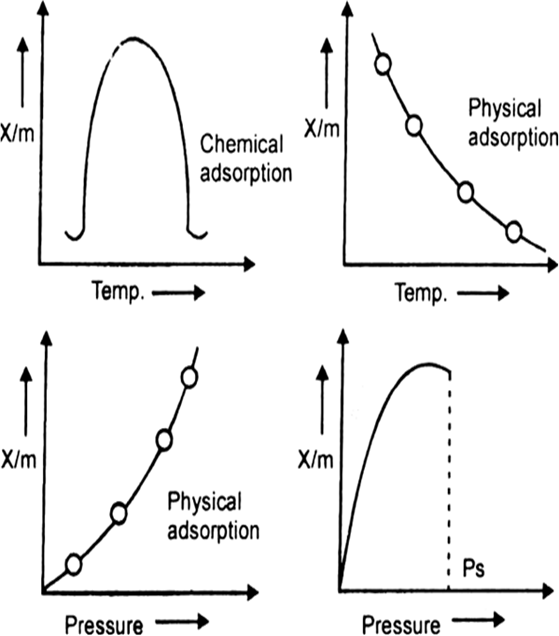
With the help of suitable diagram, describe the variation in (i) chemisorption (ii) physisorption with changes in temperature and pressure of adsorbed gas. How may these diagram be used to distinguish the two of adsorption?
 first increases and then decreases with increase in temperature it is chemical adsorption. If it decreases with increase in temperature, it is physical adsorption.
first increases and then decreases with increase in temperature it is chemical adsorption. If it decreases with increase in temperature, it is physical adsorption.If

increases with
increase in pressure, it is physical adsorption. If it first increases and then becomes independent of pressure then it is chemical adsorption.
182 Views

