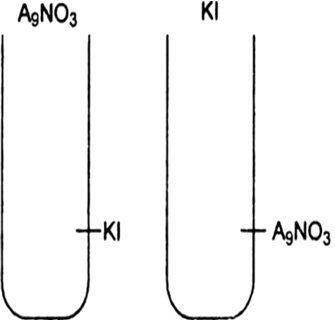
(i) What is the charge of AgI colloidal particles in the two test tube (A) and (B).
(ii) Give reason for the origin of change.
When silver nitrate solution is added to potassium iodide solution, the precipitated silver iodide adsorbs iodide ions from the dispersion medium and negatively charged colloidal solution results.
However when KI solution is added to AgNO3 solution, positively sol result due to adsorption of Ag+ ions from dispersion medium.![]()
