Why is an increase in temperature observed on mixing chloroform with acetone?
Answer:
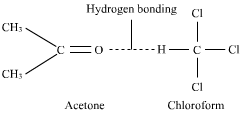
When chloroform is added to acetone there are new forces of attraction due to hydrogen bonding between acetone and chloroform.
ΔHmix is negtive because energy is released due to increase in the attractive forces, therefore dissolution is the exothermic process . hence temperature is increases.
439 Views

