Examine the illustration of a portion of the defective crystal given below and answer the following questions:
(i) What are these types of vacancy defects called?
(ii) How is the density of a crystal affected by these defects?
(iii) Name one ionic compound which can show this type of defect in the crystalline state.
(iv) How is the stoichiometry of the compound effected?
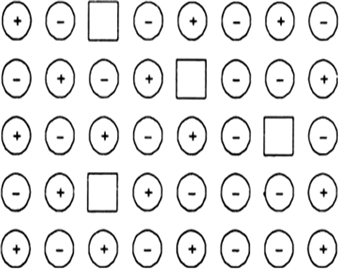
(i) These type of vacancy defects are called Schottky defects.
(ii) This defect decreases the density of the crystal.
(iii) NaCl shows this type of defect in the crystalline state.
(iv) This is the point defect which does not disturb stoichiometry of the solid.
1259 Views

