NF3 does not have donor properties like ammonia. Explain.
NF3 has a pyramidal shape with one lone-pair on N atom.
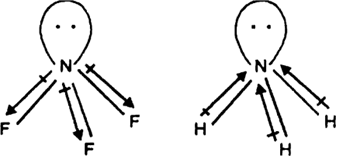
The lone-pair on N is in opposite direction to the N—F bond moments and therefore it has very low dipole moment (about 0.234 D). Thus it does not show donor properties. But ammonia has high dipole moment because its lone pair is in the same direction as the N—H bond moments. Thus it has donor properties.
The electronegtivity also influence the donar properties. fluorine is more electronegtive than hydrogen thus fluroine pull electron from nitrogen but hydrogen is not.
487 Views

