A metal ion Mn+ having d4 valence electronic configuration combines with three didendate ligands to form complex compound. Assuming ∧0 > P.
(i) Draw the diagram showing d-orbital splitting during this complex formation.
(ii) Write the electronic configuration of the valence electrons of the metal Mn+ in terms to t2g, and eg .
(iii) What type of hybridisation will Mn+ ion have?
(iv) Name the type of isomerism exhibited by this complex.
(i) Since we assuming ∧0 > P thus, ligand is weak field ligand therefore it does not caused pairing.
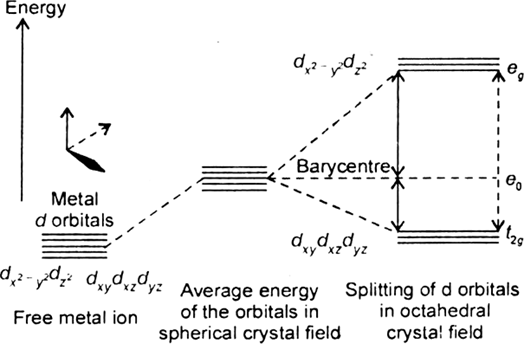
(ii) Electronic configuration is t32g, e1g because ligand is weak.
(iii) ligand is three didendate ligand therefore it contribute six electron thus it hybirdization is sp3d2
(iv) Coordination isomerism.
2376 Views

