[Fe(CN)6]4− In the above coordination complex, iron exists in the +II oxidation state. Fe2+. Electronic configuration of Fe2+is 4s0 3d6.
As CN− is a strong field ligand, it causes the pairing of the unpaired 3d electrons.
Since there are six ligands around the central metal ion, the most feasible hybridization is d2sp3. d2sp3 hybridized orbitals of Fe2+ . 6 electron pairs from CN− ions occupy the six hybrid d2sp3orbitals. Then,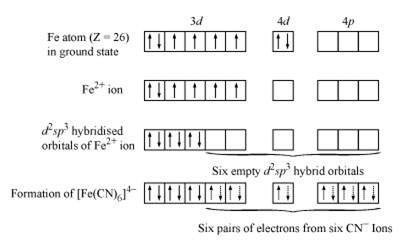
Since CN- is strong field ligand it cause pairing. hence it is diamagnetic.
In this complex, the oxidation state of Fe is +3. eletronic configuration is 4s0 3d5.
There are 6 F− ions. Thus, it will undergo d2sp3 or sp3d2 hybridization. As F− is a weak field ligand, it does not cause the pairing of the electrons in the 3d orbital. Hence, the most feasible hybridization is sp3d2.
Since [FeF6]4– have unpaired electrons. Hence it is strongly paramagnetic.
