Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species.
NiCl42-, there is Ni2+ ion, However, in presence of weak field Cl- ligands, NO pairing of d-electrons occurs. Therefore, Ni2+ undergoes sp3 hybridization to make bonds with Cl- ligands in tetrahedral geometry. As there are unpaired electrons in the d-orbitals, NiCl42- is paramagnetic. 
Since it have two unpaired electron electron therefore the magnetic moment :
In [Ni(CN)4]2-, there is Ni2+ ion for which the electronic configuration in the valence shell is 3d8 4s0.
In presence of strong field CN- ions, all the electrons are paired up. The empty 4d, 3s and two 4p orbitals undergo dsp2 hybridization to make bonds with CN- ligands in square planar geometry. Thus [Ni(CN)4]2- is diamagnetic. 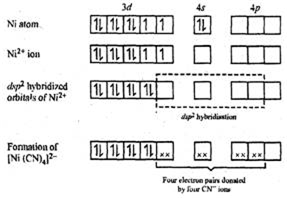
Since there is no any unpaired electron therefore its magnetic moment is zero.
