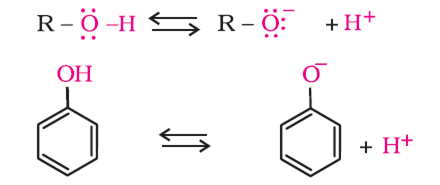
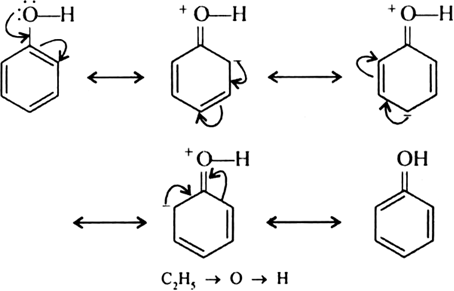
Explain why phenol has a much lower pKa value than ethanol.
In phenol, the lone pair of oxygen participates into resonance with the benzene ring.
As a result, oxygen acquires a partial positive charge. The electron density of O—H bond then shifts towards oxygen decreases around H-atom. H-atom, therefore, can easily be removed as H+ ion. While in ethanol, ethyl group has a + I effect and increases the electron density around H of O—H group making it difficult to remove H as H+. Hence, phenol is more acidic than ethanol and has lower pKa value than ethanol.