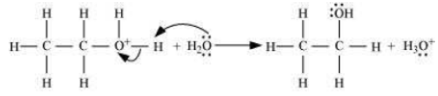
Write the mechanism of hydration of ethene to yield ethanol.
The mechanism of the reaction involves the following three step:
Step 1: Proptonation of ethene to form carbocation by electrophilic attack of H3O+.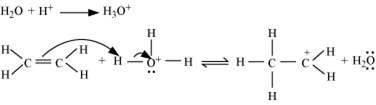
Step 2: Nucleophilic attack of water on carbocation.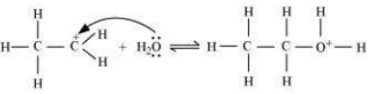
Step 3: Deprotonation to form an ethanol.